Connectomics
A Case Study of the Workhorse of Network Neuroscience
God of War is by far one of my all-time favourite video games! (Not the opener you were expecting from a science article about brain connectivity—I’m sure—but trust me, there is method to my madness…) In the latest instalment of the franchise, Kratos, Atreus, Mimir, and Freya face insurmountable odds as they confront the powerful Æsir—Odin, Thor, and Heimdall—during Ragnarök, the final battle that resets all of existence. As the story unfolds, with countless foes succumbing to his mighty Leviathan Axe, Blades of Chaos, and Draupnir Spear, Kratos learns of the imminent demise of his son from the Norns, the three goddesses of destiny who interweave the past, present, and future for all mortals and gods into a single interconnected cosmic drama. Our brains, as it turns out, also exhibit intricate patterns of connectivity that may inform how we think and act—and in this issue of ZAGROSCIENCE, we will look at connectomics, the unique analytical approach that enables us to detect the latent structural and functional associations of the cortex.
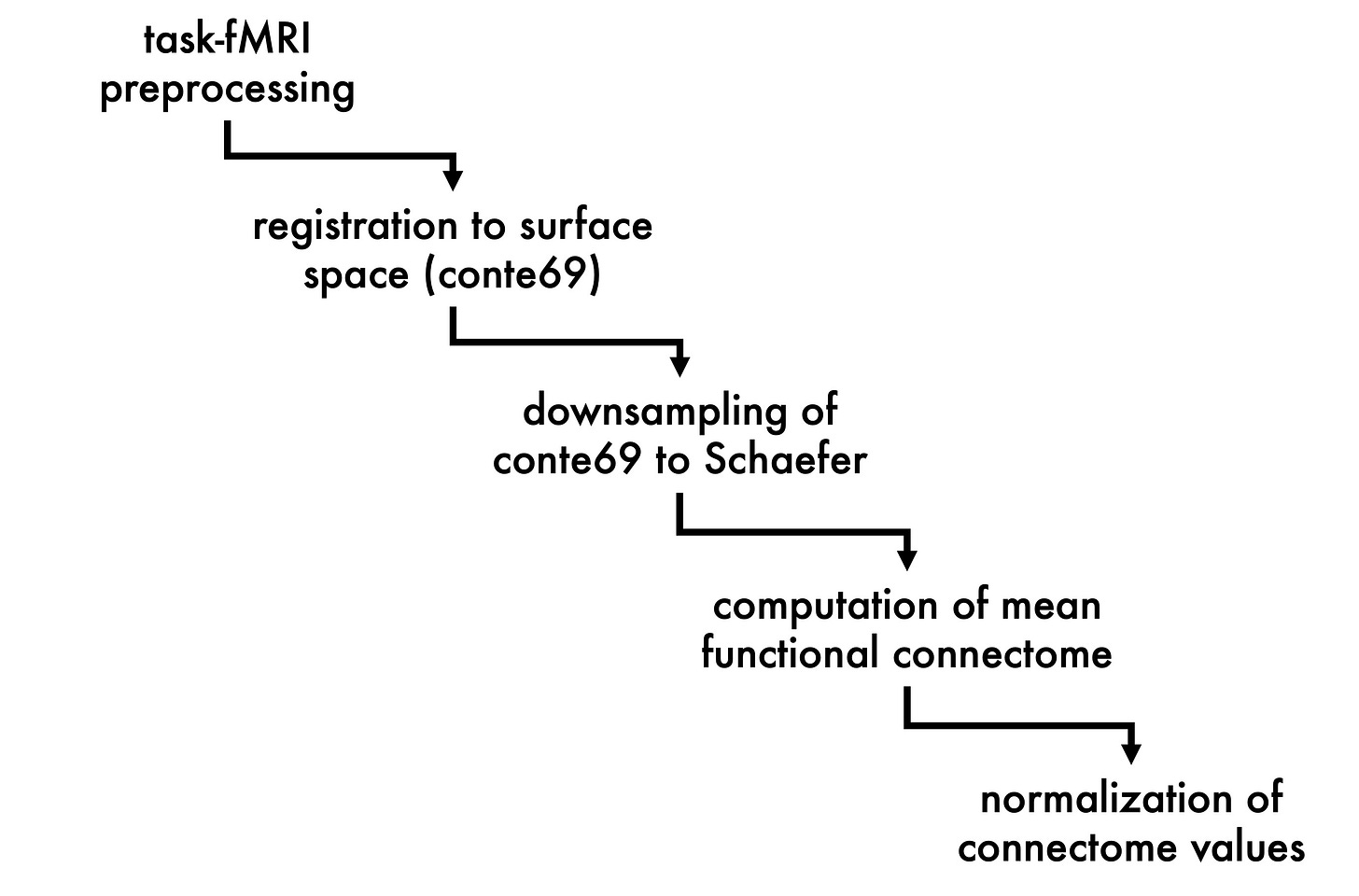
Early in my doctoral studies, I published a review (Tavakol et al., 2019) in Epilepsia by the title of “Neuroimaging and connectomics of drug-resistant epilepsy at multiple scales: From focal lesions to macroscale networks.” In it, my co-authors and I describe how the dovetailing of multimodal neuroimaging techniques and advanced connectomics has led to the characterization of pharmacoresistant epilepsy—marked by focal lesions—as a brainwide, so-called “network-level” disease. One of the findings that my review highlights is that, despite localized sclerotic lesions, disease-related brain alterations can be observed throughout the cortex. For example, in temporal lobe epilepsy (TLE) where the structures of the medial temporal lobe (i.e., hippocampus, parahippocampus, amygdala, etc.) are oftentimes affected, diffusion MRI analyses, which look at the architectural integrity of white matter tracts, have shown that microstructural anomalies that are most notable at these temporal epicentres gradually fade the farther away we look from them. MRI-based morphometrics are even more telling regarding widespread changes, with findings consistently showing “…bilateral neocortical atrophy, which affects prefrontal, lateral temporal, frontocentral, and parietal regions […]” In addition to ascertaining whole-brain structural modifications in the disease progression of TLE, connectivity analyses have also been leveraged to examine associated large-scale functional reorganization of the brain. As we note in the review, “…the severity of hippocampal structural pathology in TLE directly relates to reductions in its functional connectivity, with patients displaying marked sclerosis generally showing lower connectivity to DMN [default mode network] hubs than those with isolated gliosis […] Reduced connectivity between hippocampal and DMN hubs may contribute to more severe impairments in memory processes in those with marked hippocampal damage […]”
While my article sheds light on the significance of connectomics in identifying disease-induced structural and functional changes that go beyond the focal point of lesion, one question still remains: what actually is connectomics? To answer this question, I will walk you through a case study consisting of a specific example where I used it, myself, to explore the functional architecture of relational memory in healthy individuals and its reorganization in TLE patients.
For the sake of housekeeping, I will first define some of the terminology in alphabetical order.
connectome: the total set of structural or functional connections inside the brain.
connectomics: an analytical approach based on graph theory that is used in network neuroscience to track variations in structural and functional connectivity.
episodic memory: the mental process by which discrete events in one’s life are coalesced into an autobiographical construct known as an episode (i.e., dancing + cutting cake + unwrapping gifts → birthday party).
functional connectivity: correlational links between the activity of different anatomical regions of the brain typically derived from resting-state or task-based functional magnetic resonance imaging (rs-fMRI/task-fMRI).
graph theory: a mathematical framework used to describe the topological properties of (cortical) networks.
network: a mathematical construct consisting of nodes (or hubs) and edges (or associations) that define interconnected regions.
network neuroscience: a specialized field in neuroscience that studies cortical networks in the context of neurodevelopment, behaviour, cognition, aging, and disease via the application of connectomics.
relational memory: a catchall phrase for cognitive processes in which the constituent elements of conscious experience are bound together, typically encompassing three specific memory domains: episodic, semantic, and spatial.
semantic memory: the mental process by which facts and notions are categorized into hierarchical concepts (i.e., cat + dog + hamster → domestic pet).
spatial memory: the mental process by which physical landmarks in the vicinity and the distances that separate them are represented as an cognitive scheme (i.e., physical map → cognitive map).
structural connectivity: physical links between different anatomical regions of the brain typically derived from diffusion magnetic resonance imaging (dMRI).
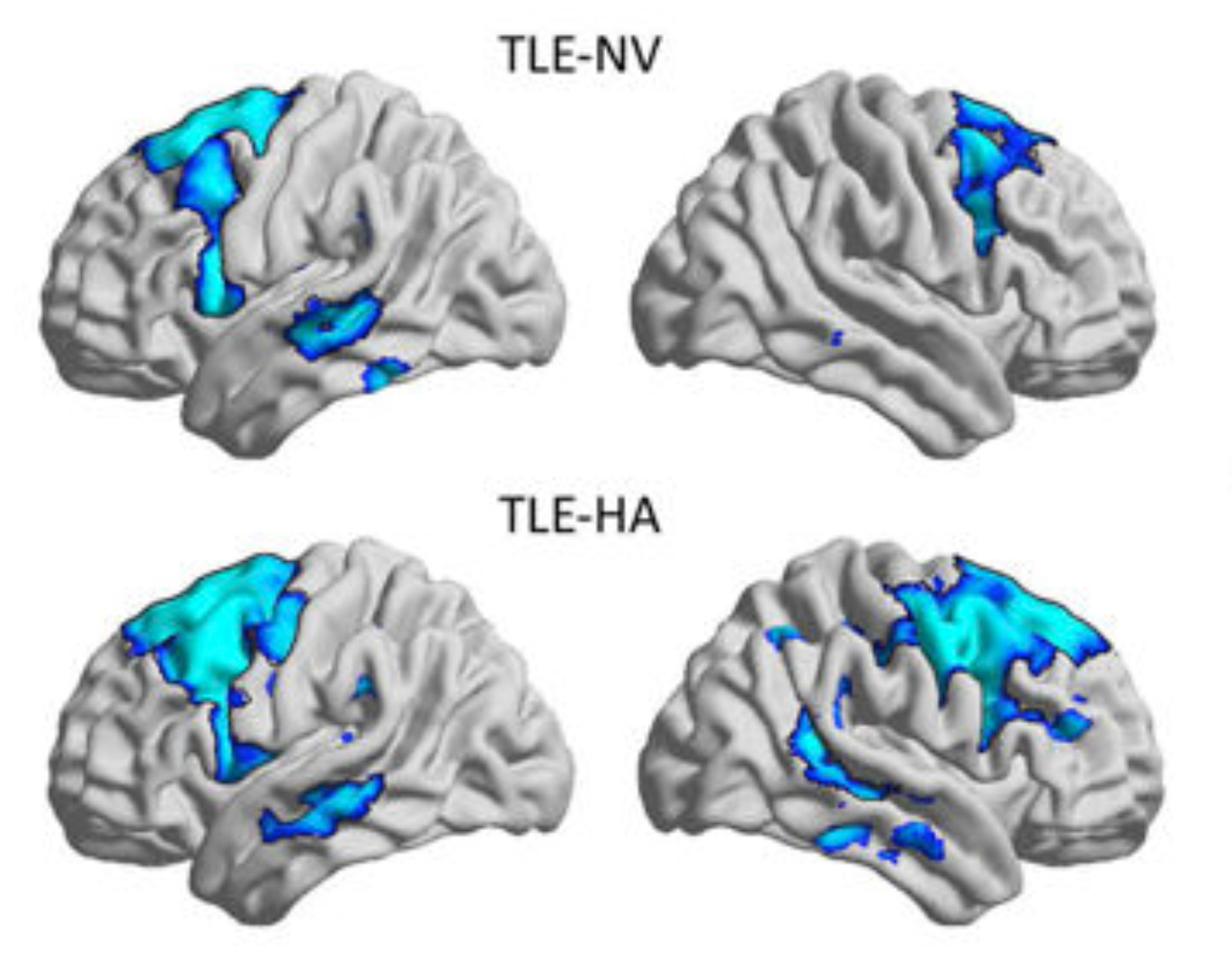
While I was pursuing my PhD at the world-renowned Montreal Neurological Institute, where Wilder Penfield and Brenda Milner laid the foundations of modern cognitive neuroscience, I developed the integrated Relational Evaluation Paradigm (iREP), the first functional MRI memory assessment protocol to combine all three domains of relational memory into a single framework (Tavakol et al., 2021/2024, see figure above). For my final project, my colleagues and I administered the iREP to a group of TLE patients and age-/sex-matched healthy controls while they were being scanned inside the MRI machine. For each subject, we computed performance scores and functional connectomes corresponding to the episodic, semantic, and spatial modules of the iREP. We then employed partial-least squares correlation analysis, a data-driven statistical approach that detects latent patterns across different datasets that maximize their shared information content. In a nutshell, we aimed to uncover hidden associations between behavioural metrics of memory capacity and functional measures of brain activity that can distinguish between healthy individuals and TLE patients. My work was ultimately motivated by the drive to understand how disease-induced alterations to the functional substrate of the brain can offset the different cognitive systems that collectively make up relational memory.
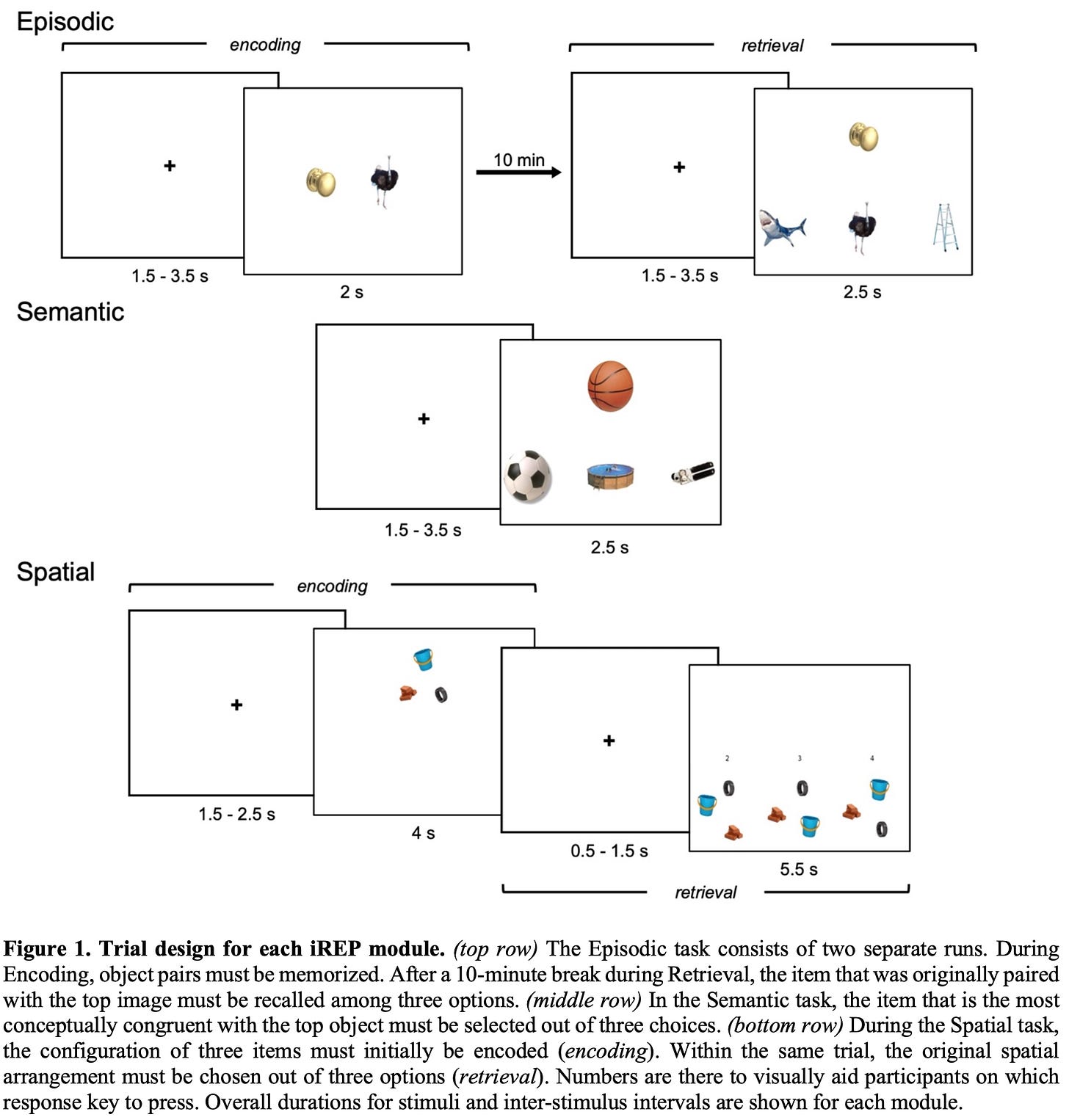
So the question now is: how did I actually derive subject-specific functional connectomes from raw task-based MRI acquisitions? The following is a step-by-step walkthrough that will help clarify the overall procedure:
Raw task-fMRI scans contain a lot noise that must be removed prior to manipulating the information they contain in any meaningful way. In a series of computational steps collectively known as data preprocessing, the effect of these confounding artifacts is considerably minimized. Sources of signal distortion typically include inhomogeneities of the magnetic field, positional changes of the participant’s head throughout the scan, drifting and heating of the mechanical components of the scanner, and baseline physiological processes like cardiac and respiratory activity.
Subject-specific preprocessed fMRI data can either be examined individually (i.e., first level) or lumped together with data from other participants for group assessment (i.e., second level). First and second level analyses can be conducted in volumetric space, which employs 3D voxels, or surface space, which utilizes point vertices. For my project, I opted for surface space analysis as it excludes partial-volume effects seen in voxels that lie at the boundary of grey and white matter. As such, surface analysis is more specific to the grey matter of the cortical mantle, which is where the metabolic activity of the brain occurs. So, for each participant, the cleaned task-fMRI time-series for the episodic, semantic, and spatial modules of the iREP were registered to a standard surface template (i.e., conte69) and computationally smoothed to increased signal-to-noise ratio.
The conte69 template contains more than 64,000 vertices, and typical iREP task-fMRI data count several hundred time points. This regimen works out to tens of millions of datapoints for a single subject on a single task…which is a lot! Due to the computational overhead that such huge data matrices present, I implemented a dimensionality reduction technique to streamline my statistical analyses, which consisted of mapping the 64+K vertices of the conte69 surface onto the 400 parcels of the Schaefer scheme (Schaefer et al., 2018), effectively downsampling the total number of features in my datasets by a factor of ~160.
I calculated mean functional connectomes for the downsampled task-specific time-series of individual subjects. I accomplished this step by cross correlating the datapoints in each of the 400 Schaefer regions with those of every other region in the parcellation, producing a 400 x 400 matrix. For each parcel, I then computed its average value across the entire scheme, yielding a 1 x 400 matrix. In the end, all participants had three 1 x 400 matrices (i.e., one for each iREP task).
I applied Fisher Z-transformation to individual mean functional connectomes to normalize the distribution of underlying correlation coefficients. This statistical approach is used to express different correlational datasets on the same scale, enabling direct comparisons. It is the parametric solution that resolves the issue of comparing apples and oranges!
By the end, my analyses uncovered hidden patterns of diseased-related brain-behaviour associations, which identified relatively older TLE patients who presented with comparatively smaller hippocampi as having an altered functional brain architecture that reflected deficits in episodic, semantic, and spatial memory.
Galen of Pergamon, the ancient Roman physician, was one the earliest to propose that neuropathologies that begin locally could spread to other parts of the cortex via the flow of animal spirits in the brain (Fortino et al., 2015). Since then, the field of connectomics has come a long way, with arguably the biggest milestone occurring in 2009 when the Human Connectome Project (HCP) was launched as part of the National Institutes of Health Blueprint for Neuroscience, aiming to outline profiles of structural and functional connectivity in healthy and clinical populations (Shah et al., 2022). Today, the HCP offers a variety of services available to the international neuroscientific community for the advancement of connectomics, including openly accessible methodological guidelines, processing and analytical softwares, and multimodal neuroimaging datasets. A simple search on PubMed for peer-reviewed articles containing the term connectivity, connectome, or connectomics in the title returns nearly 28,000 hits between the years 2009 and 2025. Indeed, connectomics represents a staple of network neuroscience that continues to inform our understanding of the topological organization of behaviour, cognition, and health inside the brain. While offering an expansive look into the inner workings of the brain, cortical connections are dynamic in nature, capable of rewiring in the face of ever-changing mental and psychological demands that the external world presents. I am reminded of what Kratos learned about the essence of fate when visiting the Norns, that the future is not yet written, that the past and present can only ever offer us a best guess of what is to come: “There is no grand design. No script. Only the choices you make. That your choices are so predictable merely make us seem prescient.” Connectivity analyses may not offer us neural prescience per se, but much like the Norns in God of War, they equip us with valuable information that can be used to anticipate the future—be it how a disease of the brain might progress or how a final showdown between Kratos and Heimdall might play out.
References
Fornito, A., Zalesky, A. and Breakspear, M., 2015. The connectomics of brain disorders. Nature reviews neuroscience, 16(3), pp.159-172.
Schaefer, A., Kong, R., Gordon, E.M., Laumann, T.O., Zuo, X.N., Holmes, A.J., Eickhoff, S.B. and Yeo, B.T., 2018. Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cerebral cortex, 28(9), pp.3095-3114.
Shah, H.A., Mehta, N.H., Saleem, M.I. and D’Amico, R.S., 2022. Connecting the connectome: a bibliometric investigation of the 50 most cited articles. Clinical Neurology and Neurosurgery, 223, p.107481.
Tavakol, S., Kebets, V., Royer, J., Li, Q., Auer, H., DeKraker, J., Jefferies, E., Bernasconi, N., Bernasconi, A., Helmstaedter, C. and Arafat, T., 2024. Differential relational memory impairment in temporal lobe epilepsy. Epilepsy & Behavior, 155, p.109722.
Tavakol, S., Li, Q., Royer, J., Vos de Wael, R., Larivière, S., Lowe, A., Paquola, C., Jefferies, E., Hartley, T., Bernasconi, A. and Bernasconi, N., 2021. A structure–function substrate of memory for spatial configurations in medial and lateral temporal cortices. Cerebral Cortex, 31(7), pp.3213-3225.
Tavakol, S., Royer, J., Lowe, A.J., Bonilha, L., Tracy, J.I., Jackson, G.D., Duncan, J.S., Bernasconi, A., Bernasconi, N. and Bernhardt, B.C., 2019. Neuroimaging and connectomics of drug‐resistant epilepsy at multiple scales: From focal lesions to macroscale networks. Epilepsia, 60(4), pp.593-604.
Note: The author “Tavakol, S.” is Shahin Zagros (myself).